How to apply reimbursement in Estonia
Information leaflet for marketing authorization holders
Dear Marketing Authorization Holder,
Here is a summary of the information on submitting an application of new and generic retail (outpatient use only distributed through retail pharmacies) medicinal products (medicines) into the Estonian list of medicinal products distributed at a discount (also referred to as reimbursement list or positive list etc).
NB! If the product is for hospital use, different rules apply. You can find more information here or you can contact EHIF for further directions.
The Estonian Health Insurance Fund (EHIF) processes the applications for new and generic medicines submitted by the manufacturers. The EHIF together with the Ministry of Social Affairs ensures that all the necessary medicines are put on the list of medicines distributed at a discount in a timely manner on a quarterly basis (on 1 January, 1 April, 1 July and 1 October), simultaneously with the adjustment of reference prices. Applications to EHIF can be submitted at any time.
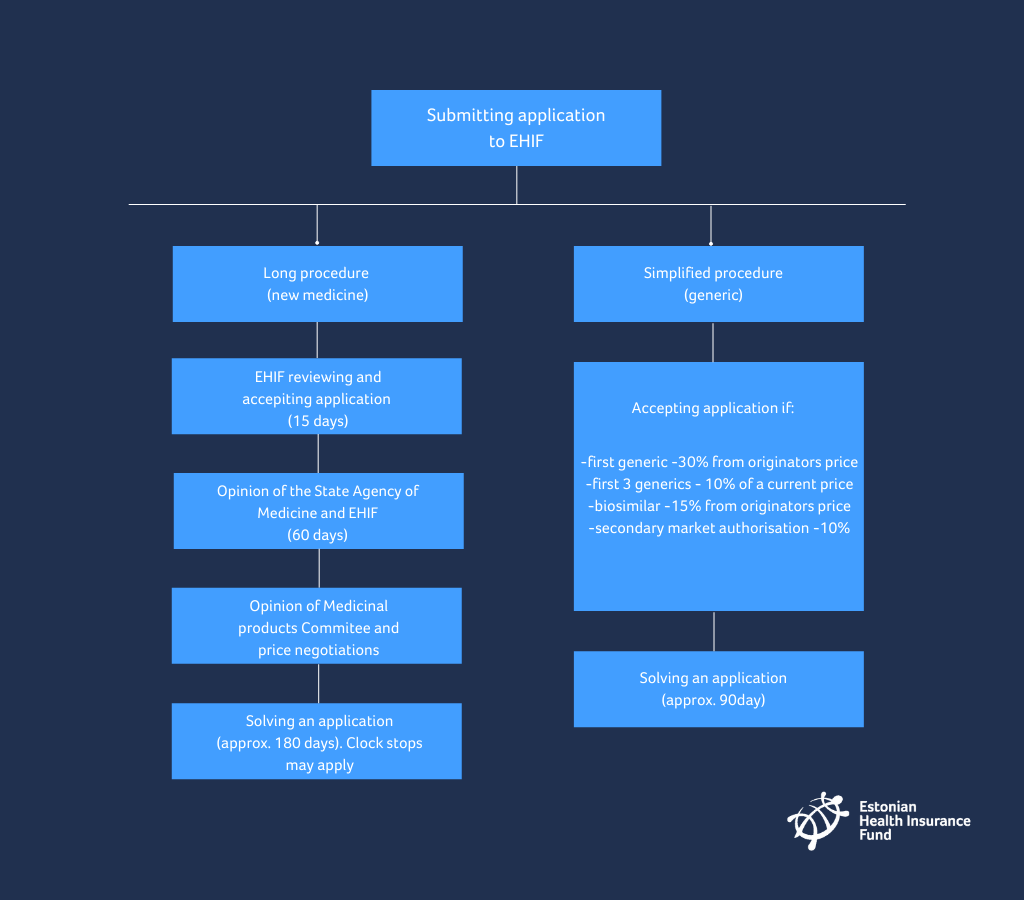
What do you have to do when you want to submit an application for a new active substance to the list of medicinal products distributed at a discount?
- An application, together with supplementary documents, has to be submitted in electronic format viewable with Acrobat Reader or Microsoft Office software, or in written format.
- If the application or it’s annexes include mathematical models (eg for cost-effectiveness analysis), the information included has to be usable with Microsoft Office software. If not, software for modelling with the necessary rights of intellectual property and other rights shall be enclosed.
- The application and annexes are in Estonian, except for copies of articles and scientific research.
- The annex to the application may be presented in English if the application concerns a medicine necessary to treat rare disease as defined in the Regulation (EC) No 141/2000 of the European Parliament and of the Council on Orphan Medicinal Products (OJ L 18, 22.1.2000, p. 1).
- description of the area of use of the product in Estonia (including the number of patients, treatment methods used, unmet medical needs, analysis regarding the possible retail sale volume of the medicinal product, and the predicted retail sale of the medicine for 3 years);
- medicinal benefits expected from the use of the product (e.g. life-saving, symptomatic, improving the quality of life, etc.) with references to scientific publications and research as well as copies to the referenced scientific publications and research;
- description of the optimal use of the product (duration, dosing etc.);
- description of side effects and their medical-economic evaluation (eg their influence on quality of life, additional test needed for the optimum use or prevention of side effects etc.);
- wholesale purchase prices of all packages of the medicine and estimated changes in the prices for the next 3 years;
- pharmacoeconomic analysis of using the medicine according to the instruction for Baltic countries published on the web page of the health insurance fund for pharmacoeconomic evaluation of medicinal products,
- a confirmation regarding the lack of additional information;
- a copy of a document proving applicant’s right of representation (Power of Attorney);
- other documents established by the legislation.
- Pharmacoeconomic analysis on the use of the medicinal product does not have to be adapted to the context of Estonia if it is a medicine used for treating a rare disease.
- The health insurance fund and the State Agency of Medicines may request additional information and documents if those are necessary for correct and quick solution of the application.
- The run of proceedings is suspended for a period not exceeding 60 days until the health insurance fund or the State Agency of Medicines receive the information and documents provided in subsection 4 of this section.
- The compliance of an application with the requirements is checked at the health insurance fund. In the event of shortcomings, a period for elimination of shortcomings shall be assigned within 15 days. The period shall not be shorter than 10 days or longer than 60 days.
- The calculation of the period of the procedure shall stop from the moment of assigning a term for elimination of shortcomings until the elimination of shortcomings by the manufacturer of the medicinal products.
- The health insurance fund submits an application to the State Agency of Medicines within 15 days.
- Applicant has a right to amend an application at his own disposal, submitting a new annex to the application to replace the original annex.
- In the event of an amendment to the application, processing terms shall be calculated from the submission of the amendments.
- The health insurance fund informs the State Agency of Medicines of an amendment to or withdrawal of the application
- The State Agency of Medicines and health Insurance fund (EHIF) prepare a written opinion on the application both together within 60 days from the arrival of the application at the State Agency of Medicines.
- Copies of the opinions are sent to an applicant. Applicant has a right for objections in written format, which will be included in the documentation pack which is sent to the Medicinal Products Committee
- The Medicinal Products Committee (hereinafter the committee) is an advisory committee of the management of the EHIF.
- Committee advises the management of the health insurance fund about addition or deletion of medicines from the reimbursement list, amendments to the reimbursement level (%) or highest acceptable price of a product, or necessary restrictions for reimbursement of a product (eg certain diagnoses only)
What do you have to do when you want to submit application for a generic active substance to list of medicinal products distributed at a discount?
- You have to fill out an application and send it to info [at] tervisekassa.ee (info[at]tervisekassa[dot]ee)
- By solving the application EHIF considers the following:
- the generic medicine is at least 30% cheaper than the originator with the same active ingredient entered into the list of medicines;
- in case of a biologically similar medicine, the fact that the said medicine is at least 15% cheaper than the originator product entered into the list of medicines;
- in case of medicine with the secondary marketing authorisation, the fact that the medicine is at least 10% cheaper than the originator product entered into the list of medicines;
- after the establishment of a reference price in a group of medicines with the same active ingredient and method of administration, the fact that the price of the medicine upon solving of the first three applications is at least 10% cheaper and upon solving of the following applications, not higher than the price of the cheapest medicine entered into the list of medicines.
Please note:
- Before making a decision, the health insurance fund may prepare a thorough opinion and request an opinion from the State Agency of Medicines and the committee.
- In the solving of an application, EHIF may ask additional information from the applicant.
The health insurance fund may request the opinion of the committee, if:
- solving of the application may have an excessive budget impact;
- the decision is of principle importance for the Estonian medicines policy.
In most cases the management of the health insurance fund solves the application within 90 days from submission of the application.
If you need more information about submission and processing of applications, it is described in the regulation of the Minister of Social Affairs: Procedure for drafting and amendment of a list of medicinal products of the Estonian Health Insurance Fund and the content of criteria establishing the list and evaluators of compliance with the criteria
